
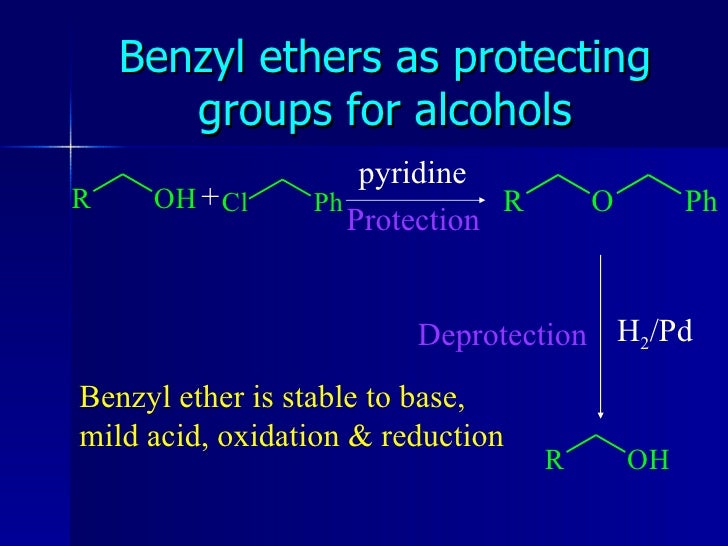
Benzyl ether (Bn)protecting group.
Common protecting groups Alcohol protecting groups Protection of alcohols : Protection of alcohol as tetrahydropyranyl ether followed by deprotection. Both steps require acid catalysts. Acetyl (Ac) - Removed by acid or base (see Acetoxy group ). Benzoyl (Bz) - Removed by acid or base, more stable than Ac group.
Protecting Groups
Use of NaH as base for the deprotonation is convenient, but when selective substitution is needed - for example, protection of one hydroxyl group in diols or selective protection of a more accessible group - mild bases such as Ag 2 O allow a more selective reaction.
BSTFA Protecting Group Chemical Compound Benzyl Group Chemical Formula, PNG, 1200x1017px
Functional Groups: Amino Carbonyl Carboxyl Hydroxyl ( 1,2-; 1,3-Diols) What are protective groups? A protective group (also referred to as "protecting group") is a reversably formed derivative of an existing functional group in a molecule.
PPT Aromatic Nomenclature PowerPoint Presentation, free download ID5172827
A list of typical conditions for benzyl deprotection. 1) Kocienski, P. J.; Protecting Groups, 3rd Edition 2) Wuts, P. G. M.; Greene, T. W.; Greene's Protective Groups.

Benzyl (Bn) ether as a protecting group for alcohols Chemistry lessons, Chemistry lecture
H. Sajiki, Tetrahedron Lett., 1995 , 36, 3465-3468. Benzyl esters of various acids can be chemoselectively cleaved on treatment with nickel boride in methanol at ambient temperature to give the parent carboxylic acids in high yields. Esters such as methyl, ethyl, tert -butyl, and trityl esters as well as benzyl ethers, tert -butyl ethers, and N.

(PDF) VisibleLightMediated Oxidative Debenzylation Enables the Use of Benzyl Ethers as
1.2 Requirements for Protecting Groups The use of a protecting group adds two steps to a synthesis: One for protection, the other one for deprotection. Both steps need to be virtually quantitative to not significantly affect the overall yield of the synthesis.

Adding Benzyl Protecting Group Mechanism Organic Chemistry YouTube
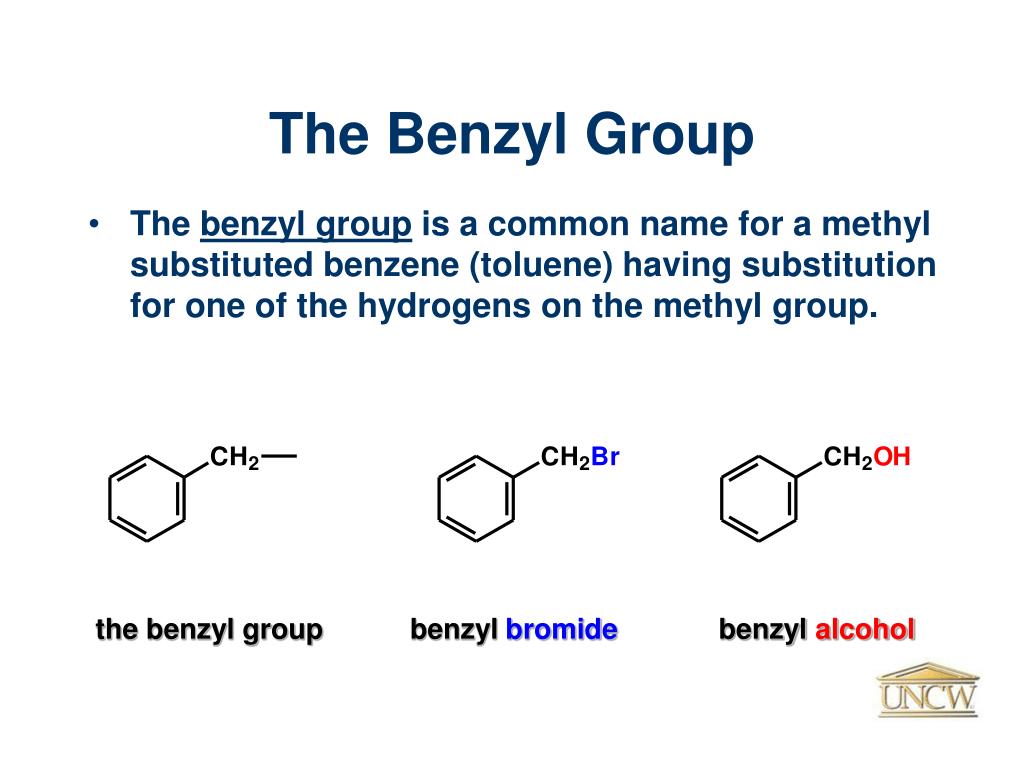
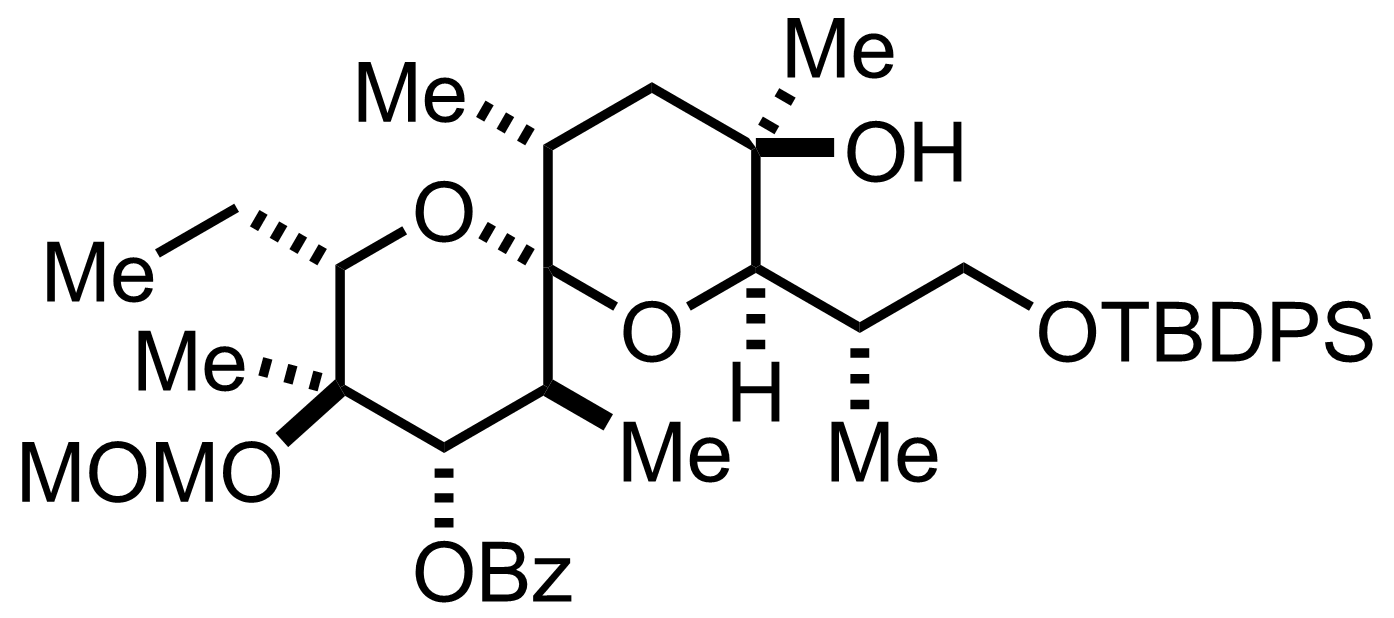
Some Common Protecting Groups in Organic Synthesis. Hydroxyl (OH) ( OH) protecting groups in Organic Synthesis. Protection of alcohols: Acetyl (Ac) ( Ac) - Removed by acid or base. Benzoyl (Bz) ( Bz) - Removed by acid or base, more stable than Ac Ac group. Benzyl ( Bn Bn, Bnl Bnl) - Removed by hydrogenolysis. Bn Bn group is widely used in.
Acid Disodium Pyrophosphate Benzyl Group Protecting Group, PNG, 720x600px, Acid, Amino Acid
A non-hydrogenolytic mild deprotection strategy for stable benzyl protection of hydroxyl group is one of the long-cherished goals in multistep synthesis involving carbohydrates or compounds with multiple hydroxyl groups. A greener organo-photocatalytic method has been developed for mild and efficient visible light catalytic.
Benzyl ether (Bn)protecting group.
75 of The Top 100 Retailers Can Be Found on eBay. Find Great Deals from the Top Retailers. eBay Is Here For You with Money Back Guarantee and Easy Return. Get Your Benzyl Today!
Phenyl vs Benzyl Groups YouTube
Every protecting group adds at least one, if not two steps to a synthesis They only detract from the overall efficiency and beauty of a route, but, without them,. cleavable groups DDQ benzyl ether p-methoxybenzyl ether. Protective Groups: Orthogonal Sets of Protecting Groups 9. Dissolving Metal Reduction OR Li/NH3, t-BuOH +ROH O OR
PPT Chapter 21, Benzene and and the Concept of Aromaticity PowerPoint Presentation ID142374
Remote Electronic Effects by Ether Protecting Groups Fine-Tune Glycosyl Donor Reactivity. The Journal of Organic Chemistry 2016, 81 (12). 1,2- cis -Selective glucosylation enabled by halogenated benzyl protecting groups. Organic & Biomolecular.
Benzyl ether (Bn)protecting group.
The benzyl group as well as its derivatives are widely adopted as protecting groups in chemical synthesis. Most of the debenzylation protocols are realized by transition-metal catalyzed hydrogenolysis or Birch reduction. However, the flammability of hydrogen and alkalis, harsh conditions, and low functional-group compatibility impede its utility.

Benzyl ether (Bn)protecting group.
Other Syntheses of Benzyl-Protected Amino Groups A highly efficient general strategy for the synthesis of 2-amino acids by homologation of α-amino acids, involving the Reformatsky reaction with a Mannich-type imminium electrophile is reported. R. Moumne, S. Lavielle, P. Karoyan, J. Org. Chem., 2006 , 71, 3332-3334. Deprotection

Benzyl ether (Bn)protecting group.
The benzyl group as well as its derivatives are widely adopted as protecting groups in chemical synthesis. Most of the debenzylation protocols are realized by transition-metal catalyzed hydrogenolysis or Birch reduction. However, the flammability of hydrogen and alkalis, harsh conditions, and low functional-group compatibility impede its utility.

(a) Cys thiol protection with the benzyl (Bn/Bzl) protecting group (b)... Download Scientific
Facile Hydrogenative Deprotection of N-Benzyl Groups Using a Mixed Catalyst of Palladium and Niobic Acid-on-Carbon Yuta Yamamoto , Eisho Shimizu , Kazuho Ban , Yoshiyuki Wada , Tomoteru Mizusaki , Masatoshi Yoshimura , Yukio Takagi , Yoshinari Sawama* , and Hironao Sajiki* Cite this: ACS Omega 2020, 5, 6, 2699-2709

reactive SN2 alkyl groups benzyl and allyl groups YouTube
As a protecting group Benzyl groups are occasionally employed as protecting groups in organic synthesis. Their installation and especially their removal require relatively harsh conditions, so benzyl is not typically preferred for protection.